Peptide receptor-mediated Radionuclide Therapy (PRRT) - Radioreceptor Therapy
What are neuroendocrine neoplasia or tumors (NEN or NET; previously: carcinoid)?
Neuroendocrine tumors are rare tumors (0.5-1/100,000 inhabitants and year) that arise from hormone-producing cells and are often able to produce hormones themselves. It is largely unknown why hormone-producing cells are induced to degenerate into tumors. NET is usually formed in the digestive system such as the stomach, small intestine, large intestine and pancreas, but also often in the lungs, thyroid and thymus.
Common symptoms are diarrhea, seizure-like flushing and sweating, asthma, etc., due to the tumor’s ability to produce hormones itself. NET is characterized by slow growth, which often causes late symptoms in the human body. However, carcinoids can be detected and treated at an early stage and can be completely cured.
What is PRRT (peptide-mediated radionuclide therapy)?
Primarily a NET should be surgically removed, if possible. In the inoperable stage there are other forms of therapy such as PRRT.
Many years ago, it was discovered that NET cells (or carcinoid cells) are characterized by a massive presence of somatostatin receptors (certain cellular "adhesive" that have signal receptive functions) on the cell surface and this property is used in PRRT.
Certain peptides are developed which bind to these adhesions . For PRRT these peptides are linked (via a chelator, usually DOTA) with a radioactive nuclide, Lutetium-177 (half life: 6.7 days). The radionuclide Lu-177 sends out ionizing radiation, with therapeutically effective ß-rays and with gamma radiation, which leaves the body. Since ß-rays have a very small range of a few millimeters in the tissue, the high radiation is limited to the cancer cells, which are thereby killed. The gamma rays make it possible to document the storage of the radiopharmaceutical in the tumor by means of a gamma camera. For radiation protection reasons, the therapy is combined with an inpatient stay at the nuclear medicine therapy ward; the standard length of stay is 5 days.
Case Report I PRRT
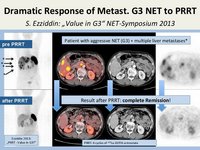
A 45y old woman with advanced G3 pancreatic neuroendocrine tumor (NET grade 3) with hepatic metastases. Patient had progressive disease after chemotherapy (etoposide/cis-platin). A dramatic reduction of tumor burden was noted after six courses of Lutetium-177 peptide receptor radionuclide therapy (PRRT) using 177Lu DOTATATE. Therapy monitoring was performed by 68Ga DOTATOC PET imaging.
For whom is PRRT suitable?
In principle, any patient with inoperable NET can be treated with PRRT. Within the framework of interdisciplinary tumor conferences, internal specialists (gastroenterologists, oncologists), surgeons, interventional radiologists, pathologists, radiation therapists and nuclear medicine physicians advise based on the available examination findings (incl. CT, MRI, PET/CT) what is the best possible therapy for a patient.
How do I get prepared for PRRT?
To avoid the blockage of the somatostatin receptor sites with the frequently used medication Sandostatin, depot preparations should be discontinued up to 6 weeks before therapy. Somatostatin receptor sites on the tumor cells are essential for the execution of peptide receptor-mediated radionuclide therapy. Information on this can be obtained by carrying out a 68-Ga-DOTA octreotide PET/CT, which is used to determine the amount of adhesive on the tumor cells.
Additionally, comprehensive laboratory diagnostics are performed as well as an assessment of renal function by means of a renal scintigraphy.
What is the therapy like?
You will be admitted to our therapy ward RN-01 the day before the therapy. Please bring a current inpatient referral certificate issued by your family doctor. The examinations for preparatory diagnostics are carried out on the day before the therapy. Prior to administration of the treatment dose, you will receive medication against nausea and an inhibitor of gastric juice.
Accompanied with the treatment dose you will receive an infusion with a medication to protect your kidneys. This is followed by the actual therapy, whereby the radioactively labeled drug is administered intravenously via infusion over approx. 30 minutes. The therapy takes place in the presence of nursing staff and the ward physician.
Following therapy, you should consume sufficient fluid to eliminate the radioactivity that was not bound to the tumor cells as quickly as possible. This can reduce the radiation exposure of your kidneys. Several scintigraphic diagnoses are performed with the Gamma camera during your inpatient stay to document the storage of the radiopharmaceutical reaching the tumor.
Case Report II PRRT
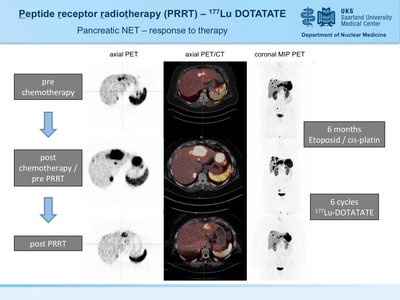
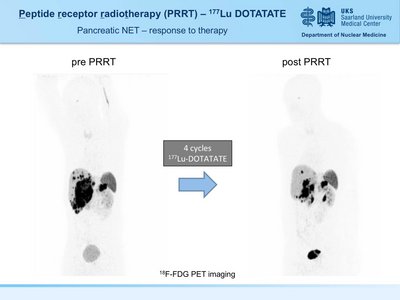
A 34y old male patient with G3 pancreatic neuroendocrine tumor (grade 3) with hepatic metasteses. Disease progression was noted after chemotherapy (5-FU/STZ). Partial remission was achieved after 4 courses of peptide receptor radionuclide therapy (PRRT) using 177Lu DOTATATE. Therapy monitoring was performed by 18F-FDG PET imaging.
What happens during the remaining inpatient stay?
The inpatient stay lasts five days. Patients are admitted on a Wednesday and released on the Monday of the following week.
Following therapy, you must not leave the controlled area, i.e. the ward RN-01, for 24 hours. On Friday, the remaining activity in your body is measured and scintigraphic images are taken with a gamma camera to document the distribution of the radiopharmaceutical in your body. Thereafter, you may leave the ward for a walk or a meeting with your family.
At least 48 hours after therapy has been completed, you will remain in the therapy ward. You can then be discharged as soon as the radioactivity in your body falls below the legal limit. On the day of discharge, the remaining radioactivity in your body is measured. This is followed by the final diagnostic scintigraphy and a final consultation with the ward physician.
What are the side effects?
PRRT is usually tolerated very well without significant side effects on the patient. Occasionally fatigue, nausea, circulatory and breathing problems, flushing symptoms or abdominal discomfort may occur. These complaints can be well treated with medication. Other possible side effects are changes in blood count and minor alterations of the liver and kidneys functions.
How often is the PRRT carried out?
PRRT is usually administered in four individual cycles at intervals of about two to three months, which enables us to administer the target therapy dose gently. Before each therapy cycle, we repeat the preparations described above, which are necessary for the therapy.
Under PRRT, what follow-up is required?
Every 14 days after therapy, the blood count, liver and kidney function should be checked by the general practitioner or treating oncologist. In addition, a MAG3 kidney function test is performed 8 weeks after therapy.
Restaging:
Approximately 2-3 months after completion of the four PRRT cycles, PET/CT scan with 68-Ga-DOTA is performed in our department to assess the success of the therapy.
Conclusion:
PRRT is an elegant procedure for targeted tumor therapy. With one infusion all tumor lesions in the body can be reached simultaneously and irradiated from the inside. It represents a promising therapeutic option in neuroendocrine tumors with few side effects!
Case management
Tel.: +49 6841 1624594
Fax: +49 6841 161724594


 uks.eu
uks.eu